Vaccine Guidelines – Risk-Based

Risk-Based Vaccines
Knowing the risk of particular diseases in your area or the area to which you are planning to travel with your horse, can help you and your veterinarian decide on which vaccinations are appropriate for your horse. The following websites provide disease information in particular areas of the world. The diseases mentioned are only those reported and generally underestimate the level of disease:
Equine Disease Communications Center – Disease Alerts (US and Canada)
Canadian Animal Health Surveillance System
Diseases for which the vaccines are risk-based/optional are those that;
- have variable impacts on the health of the animal
- often have low risk of causing life-threatening disease
- the vaccine(s) is less than perfect in its ability to prevent disease (due to the biology of the agent)
- occur primarily in a specific regional or geographic area
- a management factor significantly increases the risk of developing the disease, e.g., botulism and feeding silage
Equine influenza
Equine influenza is a contagious, respiratory disease caused by two distinct subtypes, H7N7 and H3N8, of the influenza A virus. Only influenza subtype H3N8 has been isolated over the last 20 years worldwide therefore vaccination with an H3N8 vaccine (with the most up-to-date North American strains) is recommended. To understand what strains of influenza are circulating worldwide and the current recommendations for vaccines go to OIE Expert Surveillance Panel on Equine Influenza Vaccine Composition – OIE Bulletin.
Equine influenza should be administered primarily:
- to horses 1 to 5 years of age, since they seem to be more susceptible to the disease and;
- in situations where there are frequent contacts with large numbers of horses, e.g., new arrivals to the barn/track, attendance at shows. While vaccination does not necessarily prevent influenza due to the change in strains (antigenic drift), the disease in vaccinated horses is less severe, the clinical course is shorter and there is less virus shed from infected horses thereby reducing spread of the virus.
At risk horses should be vaccinated at a minimum annually, and for some horses every 6 months when the risk of exposure continues. Usually influenza is combined with EHV-1/4 in the vaccine so more frequent vaccination is not recommended due to the combination.
Equine herpesvirus (EHV)
Equine herpesvirus was previously known as equine viral rhinopneumonitis. There are numerous strains of Equine herpesvirus (EHV) that can be transmitted between horses by body fluids, including nasal secretions. It is estimated that approximately 70% of the horse population is infected with equine herpesvirus-1 (EHV-1) which most likely occurred at birth or during the first few months of life. This virus lays dormant or asleep in a nerve cell body in the head and also in some lymph nodes. It can awaken during times of stress or illness much like the shingles virus can in people. It is then shed to horses in close proximity. In broodmares it can cause abortion, and in all horses it can cause respiratory or neurological disease. Vaccines contain EHV-1 and EHV-4 and when administered to horses provide them with some protection against the respiratory and abortion forms of the disease. No vaccine is presently labelled for protection against the neurological form of the disease.
Pregnant mares should be vaccinated with a killed EHV-1 vaccine prior to breeding if they have never received an EHV vaccine, and in the 5th, 7th, and 9th months of pregnancy.
Horses older than a year should be vaccinated one or twice a year based on risk. As with influenza, given the highly contagious nature of the disease due to equine herpesvirus 1 and 4 and the impact on horse health and industry economics, some racing regulators and racetracks as well as sport organisations have rules requiring the administration of an EHV-1,4 vaccine.

Strangles
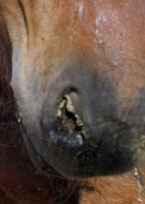
Strangles is a highly contagious and serious infection of horses and other equids caused by the bacterium, Streptococcus equi. The disease is characterized by severe inflammation of the mucosa of the head and throat, often with extensive swelling and rupture of the lymph nodes below and behind the jaw, which produce large amounts of thick, creamy pus.
Fever is an initial sign of Strangles. There is a brief window (1-2 days) before the horse starts shedding the bacteria from the nose, contaminating the environment, and infecting other horses. Swift isolation and control measures are important.
Large outbreaks of Strangles are more common in animals less than five years of age and especially in groups of weanling foals or yearlings. Horses of any age can become infected, however, and in Ontario, Strangles has been reported in horses 2 months to 26 years of age.
Currently, there are both injectable (killed vaccine) and intranasal (modified live vaccine) vaccine products available in Canada. This vaccine is administered to competition and other horses at risk of exposure to disease. The risk of exposure increases at co-mingling sites such as shows and auctions. Some vaccinated horses will show mild signs of Strangles when exposed to the bacterium and, like with other vaccines, the shedding is much reduced limiting the potential for an outbreak. The intranasal Strangles vaccine should not be administered at the same time as other injections as there is a potential for contamination of those injection sites and subsequent abscessation. As well, in rare cases, horses vaccinated with the intranasal vaccine have developed mild, noncontagious, signs of Strangles shortly after vaccination. Horses that have been administered the intranasal vaccine may also test positive on PCR testing for up to 60 days. Rarely horses vaccinated with either vaccine may develop a condition called purpura hemorrhagica. Performing an SeM titre before vaccination can identify those horses at risk for developing this complication.
Horses should receive a primary vaccination series of two to three doses, depending on the vaccine used and based on the manufacturer’s recommendations. Horses should then be vaccinated annually according to risk of exposure.

Potomac horse fever (PHF)
Potomac horse fever (PHF) is caused by the bacteria Neorickettsia ristici, and, in Ontario by a newly identified organism Neorickettsia findlayensis. It is maintained in nature in a complex aquatic ecosystem. Transmission to horses can occur through accidental ingestion of insects, such as caddisflies, damselflies, dragonflies, and stoneflies, containing Neorickettsia sp. The vaccine is made from a single strain of N. risticii therefore there has been a history of vaccine failure. Anecdotally, veterinarians in endemic areas for PHF feel that vaccination reduces the severity of disease in many situations.
After a primary two-dose series, horses should be vaccinated annually in areas where PHF has been previously diagnosed and where veterinarians have seen a positive response to vaccination. Your veterinarian will have an appreciation for the efficacy of the vaccine in your area.
Ontario Animal Health Network – Potomac horse fever fact sheet

Botulism
Botulism is a disease that occurs when toxins produced by the bacterium, Clostridium botulinum, enter the horse’s body causing weakness, which may progress to an inability to swallow, paralysis and death. The Botulism bacterium is a spore-forming anaerobic bacteria (grows in the absence of oxygen) which can occur in decaying plant material. The bacteria may also grow in wounds or in the intestinal tract of foals, releasing toxins. Neurotoxin serotypes A, B and C are associated with most Botulism outbreaks.
Horses are the most sensitive of the domesticated animals to Botulism. Hay and especially haylage or silage can be contaminated with the bacteria during the raking and baling process when animal carcass remnants may be incorporated in the forage. Hay silage can be a great feed when preserved properly but carries the danger of Botulism.
A toxoid vaccine against C. botulinum serotype B is available and should be used three times initially, 4 weeks apart, followed by an annual booster if haylage/ silage is going to be fed. The vaccine protects against type B Botulism only and foals and horses continue to be susceptible to serotypes A and C. Foals can be especially vulnerable. Discuss the need for this vaccine with your veterinarian.

Equine viral arteritis (EVA)
Equine viral arteritis (EVA) was first identified in 1953 following an extreme respiratory-abortion syndrome on a standardbred farm in Ohio.
EVA causes panvasculitis (inflammation of the veins and arteries) that results in edema of the limbs and an urticaria-like reaction (allergic/hive-like) of the head, neck and trunk.
After an incubation period of 3-14 days, clinical signs can include any combination or all of the following:
- anorexia
- fever up to 41°C for 1-9 days
- depression
- limb edema, especially of the hind limbs, scrotum and sheath
- stiffness of gait
- nasal and ocular discharges
- skin rash
- abortion in the mare
- infrequently, respiratory distress, coughing and diarrhea in the young foal
EVA can result in the establishment of the carrier state with shedding of virus into the semen in a significant percentage of infected stallions. A positive titre (either from natural infection or vaccination), to this virus may prevent a horse or its semen from being exported to another country.
Prior to vaccination, refer to importation guidelines (of the destination country) should exportation be contemplated.
Please note: This information provides guidelines only and should never replace information from your veterinarian.
References:
Guidelines for The Vaccination of Horses. Lexington; AAEP, 2008
For more information, contact
OMAFRA
Toll Free: 1-877-424-1300
Local: (519) 826-4047
E-mail: [email protected]